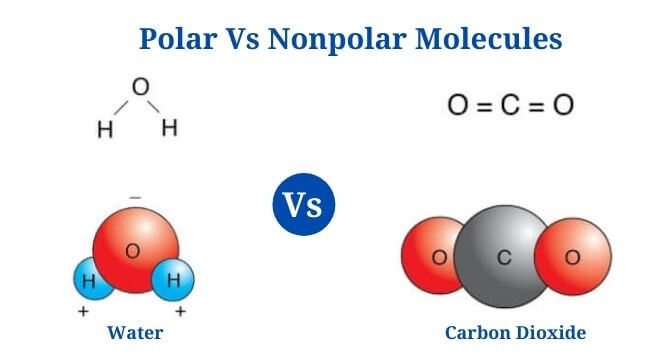
Polar Vs Nonpolar Molecules: Definition, Differences, Examples
Polar Molecules Definition A polar molecule is a chemical compound with an unequal electron distribution between the atoms, resulting in a dipole moment. Polarity refers to the difference between a molecule’s electrical poles, that indicates how polar the molecule is. The sum of all the bonds in a molecule determines whether or not the molecule … Read more