Acid Definition Chemistry (Define Acid)
The term acid and base have been defined in different ways, depending on the particular way of looking at the properties of acidity and basicity. Arrhenius first defined acids as compounds which ionize to produce hydrogen ions, and bases as compounds which ionize to produce hydroxide ions. According to the Lowry-Bronsted definition, an acid is a proton donor and a base is a proton acceptor.
Base Definition Chemistry (What is base?)
The ionic compounds that produce negative hydroxide (OH−) ions when dissolved in water are called bases. A compound containing negative nonmetal ion as well as a positive metal ion that is held together by the ionic bond is called an ionic compound.
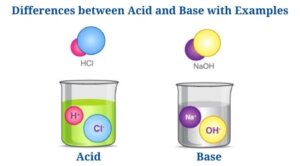
Differences between Acid and Base with Examples
(Acid vs Base)
[ninja_tables id=”5420″]
Acid and Base Citations
- https://brainly.com/question/2624790
- https://www.microchemicals.com/products/etchants.html
- https://quizlet.com/136496265/chapter-18-flash-cards/
- https://en.wikipedia.org/wiki/Acid%E2%80%93base_reaction
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_A_Molecular_Approach_(Tro)/16%3A_Acids_and_Bases/16.04%3A_Acid_Strength_and_the_Acid_Dissociation_Constant_(Ka)
- https://brainly.com/question/3458136
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example