Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
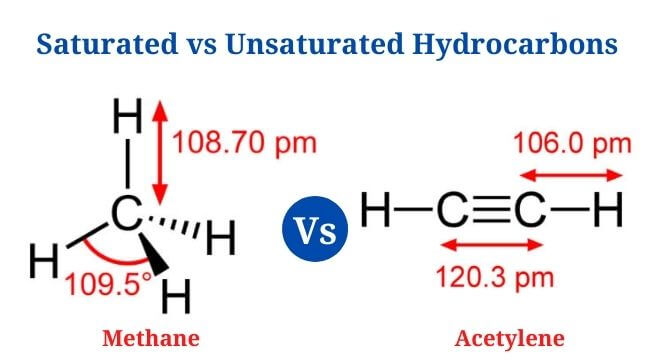
Definition of Saturated Hydrocarbons Saturated hydrocarbons are the most basic types of hydrocarbons, consisting completely of single bonds that remain hydrogen-saturated. Acyclic saturated hydrocarbons, often known as alkanes, have the general formula CnH2n+2The more general formula is CnH2n+2(1-r) , where r is the number of rings. There are no double or triple bonds between the … Read more