Table of Contents
Definition of Lipids
- Lipids are a diverse collection of chemical molecules that are soluble in non-polar organic solvents but insoluble in water.
- They are employed as cell membrane components, energy storage molecules, insulation, and hormones in most plants, animals, and microbes.
Lipids Characteristics
- At room temperature, lipids can be either liquids or non-crystalline solids.
- Colorless, odourless, and tasteless are the characteristics of pure fats and oils.
- They’re organic compounds with a lot of energy.
- It is water insoluble.
- Alcohol, chloroform, acetone, benzene, and other organic solvents are soluble.
- There are no ionic charges.
- Saturated fatty acids are abundant in solid triglycerols (fats).
- Unsaturated fatty acids are abundant in liquid triglycerols (oils).
1 . Triglycerol hydrolysis
Hydrolysis is the process through which glycerols, like any other esters, react with water to generate their carboxylic acid and alcohol.
2. Saponification
There are numerous ways to hydrolyze triacylglycerols, the most common of which uses alkali or lipase enzymes. Saponification is the name given to alkaline hydrolysis since one of the products is a soap, usually sodium or potassium salts of fatty acids.
3. The process of hydrogenation
Unsaturated fatty acids’ carbon-carbon double bonds can be hydrogenated by interacting with hydrogen to form saturated fatty acids.
4. Use of halogens
Unsaturated fatty acids react with halogens by adding at the double bond, whether they are free or mixed as esters in fats and oils (s). The halogen solution is decolorized as a result of the reaction.
5. Toxicity
Any fat or oil that has an unpleasant odour is referred to as rancid. The causes of rancidity are hydrolysis and oxidation processes. In triacylglycerols containing unsaturated fatty acids, oxidative rancidity occurs.
Lipids Structure
- Lipids are formed up of the elements Carbon, Hydrogen, and Oxygen, although they contain far less water than other molecules like carbohydrates.
- Lipids are not polymers, unlike polysaccharides and proteins, because they lack a repeating monomeric unit.
- Glycerol and fatty acids are the two chemicals that make them up.
- Three carbon atoms are connected to a hydroxyl group, and hydrogen atoms fill the remaining spots in a glycerol molecule.
- Fatty acids are made up of an acid group on one end of the molecule and an R-shaped hydrocarbon chain on the other.
- It’s possible that they’re saturated or unsaturated.
- There are no C=C bonds in a saturated fatty acid since every potential bond is created with a hydrogen atom.
- C=C bonds do exist in unsaturated fatty acids, on the other hand. Polyunsaturated fatty acids have more than one C=C bond, whereas monounsaturated fatty acids have only one C=C bond.
Triglycerides’ Structure
- Triglycerides are lipids made up of one glycerol molecule and three fatty acid molecules linked together.
- The covalent bonds between the molecules are known as Ester bonds.
- They develop as a result of a condensation reaction.
- Because the charges are equally distributed throughout the molecule, hydrogen bonds with water molecules do not form, rendering the molecule insoluble in water.
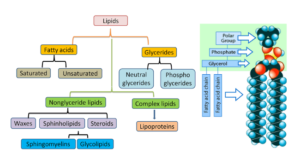
Lipid Types and Classification
Lipids can be divided into groups based on their hydrolysis products and molecular structure similarities. There are three major subclasses:
1 . Lipids in their purest form
(a) Fatty acids and glycerol are produced when fats and oils are hydrolyzed.
(b) Waxes, which when hydrolyzed generate fatty acids and long-chain alcohols.
Oils and Fats
- Because they are esters made of three fatty acids attached to glycerol, trihydroxy alcohol, both types of compounds are referred to as triacylglycerols.
- The distinction is based on their respective physical states at room temperature. If a lipid is solid at 25°C, it is called fat; if it is a liquid at the same temperature, it is called oil.
- Different degrees of unsaturation of the constituent fatty acids are reflected in the melting points.
Waxes
- Wax is an ester comprising a fatty acid and a long-chain alcohol (typically mono-hydroxy).
- Acids and alcohols often found in waxes contain carbon chains ranging from 12 to 34 carbon atoms long.
2. Compound Liquids
- When phospholipids are hydrolyzed, they produce fatty acids, glycerol, the amino alcohol sphingosine, phosphoric acid, and nitrogen-containing alcohol.
Depending on the alcohol group present, they can be glycerophospholipids or sphingophospholipids (glycerol or sphingosine).
(b) Glycolipids, which when hydrolyzed generate fatty acids, sphingosine, or glycerol, and a carbohydrate.
Depending on the alcohol group present, they can alternatively be called glyceroglycolipids or sphingoglycolipids (glycerol or sphingosine).
3. Derived lipids
- Derived lipids are the hydrolysis products of simple and complex lipids. Fatty acids, glycerol, sphingosine, and steroid derivatives are among them.
- Steroid derivatives are phenanthrene structures that are distinct from fatty acid-based lipids.
Functions of Lipids
It is well documented that lipids play a critical part in a cell’s regular activities. Lipids not only serve as significantly reduced energy storage forms, but they also play an important function in the formation of cell and organellar membranes. Lipids serve a variety of purposes, including:
- Storage of energy
- Biological Membrane Fabrication
- Insulation
- Protection – e.g. protecting plant leaves from drying up
- Buoyancy
- Hormone-like effects
- Act as a structural component of the body, forming a hydrophobic barrier that allows the aqueous contents of cells and subcellular structures to be partitioned.
- Lipids are an important source of energy in animals and crops with a high lipid content.
- Phosphatidylcholine micelles are required for the activation of enzymes such as glucose-6-phosphatase, stearyl CoA desaturase and -monooxygenase, and -hydroxybutyric dehydrogenase (a mitochondrial enzyme).
Click Here for Complete Biology Notes
Lipids Citations
- http://www.phys.sinica.edu.tw/TIGPNANO/Course/2006_Spring/classnotes/Nanobio%20031006.pdf
- http://www.notesonzoology.com/lipids/lipids-definition-classification-an-functions-biochemistry/3510
- https://alevelnotes.com/Lipids/58
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
Related Posts