What is Benedict’s Test ?
Benedict’s test is a simple chemistry test used to detect reducing sugars. Reducing sugars are carbohydrates having free aldehyde or ketone functional group in its molecular structure.
Objectives of Benedict’s Test
- To determine the presence or absence of reducing sugar in the solution.
- To determine the glucose concentration in the solution quantitatively.
Principle of Benedict’s Test
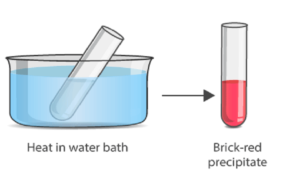
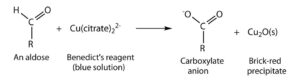
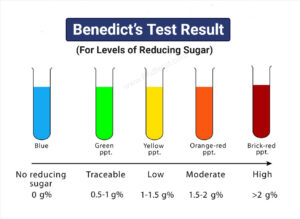
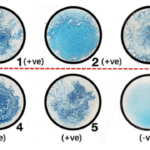
Benedict’s test is performed by heating the reducing sugar with Benedict‘s reagent. The presence of the alkaline sodium carbonate converts the sugar into a strong reducing agent called enediols. During the reduction reaction, the mixture will change its color from blue to brick-red precipitate due to the formation of cuprous oxide (Cu2O). Copper in its cupric (Cu2+) or copper (I) form is reduced to cuprous (Cu+) or copper (II). The red-colored cuprous oxide is insoluble in water and hence, separated. If the concentration of the sugar is high, then the color becomes more reddish, and the volume of the precipitate increases.
Composition and Preparation of Benedict’s reagent
One litre of Benedict’s Solution can be prepared from 100 g of anhydrous sodium carbonate, 173 g of sodium citrate and 17.3 g of copper(II) sulfate pentahydrate.
Quality Checking : Benedict’s solution is blue in color. In order to check purity of Benedict’s solution take 5 ml of Benedict’s solution in test tube and heat it. If is does not change color, it means it is pure.
Procedure of Benedict’s Test
- Approximately 1 ml of sample is placed into a clean test tube.
- 2 ml (10 drops) of Benedict’s reagent (CuSO4) is placed in the test tube.
- The solution is then heated in a boiling water bath for 3-5 minutes.
- Observe for color change in the solution of test tubes or precipitate formation.
Benedict’s Test Observation /Results)
Limitation of Benedict’s Test
The limitations of Benedict’s test are as follows:
- False-positive reactions in the test can also be obtained if there are certain drugs present for example, salicylates, isoniazid, streptomycin, penicillin, and p-aminosalicylic acid.
- The chemicals present in the concentrated urine may reduce Benedict’s reaction which includes urate, creatinine, and ascorbic acid (the reduction is slight).
Benedict’s test Citations
- National Biochemicals Corp.- BENEDICT’S SOLUTION (MB4755).
- Science Olympiad- Use of Benedict’s Solution.
- Brilliant Biology Student 2015- Food Tests- Benedict’s Test for Reducing Sugars.
- BBC Bitesize- Chemistry- Carbohydrates.
- University of Manitoba- The Molecules of Life: Biochemistry- Carbohydrates.
- Northern Kentucky University- Benedict’s Reagent: A Test for Reducing Sugars.
- KNUST Open Educational Resources, Benedict’s Test – Qualitative Test in Carbohydrates.
- Mark Rothery’s Biology Web Site- Biochemical Tests.
Related Posts
- Anisocytosis: Definition, Types, Causes, Symptoms, Treatment
- Endospore Staining: Principle, Procedure, Reagents, Results
- Flow Cytometry: Overview, Principle, Steps, Types, Uses
- Northern Blot: Overview, Principle, Procedure and Results
- MPV Blood Test: Calculation, High and Low MPV Value, Results
- Latex Agglutination Test: Objectives, Principle, Procedure, Results
- Iodine Test: Definition, Objective, Principle, Procedure, Results
- Eosin Methylene Blue (EMB) Agar
- Biuret Test for Protein: Purpose, Objectives, Principle, Procedure, Reagents
- Streak Plate Method: Meaning, Principle, Methods, Importance, Limitations
- Bile Solubility Test: Objective, Principle, Procedure, Results, Uses
- Butyrate Disk Test: Objective, Principle, Procedure, Results, Uses, Limitations
- Beta Lactamase Test: Objective, Principle, Procedure, Results, Limitations
- Bacitracin Susceptibility Test: Objective, Principle, Procedure, Results, Uses, Limitations
- Bile Esculin Test: Objective, Principle, Procedure, Result, Uses, Limitations