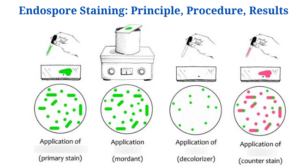
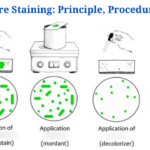
Endospore Staining-Introduction
A vegetative form of a bacterial cell is a normal bacterial cell. It may multiply indefinitely if given favourable environmental circumstances like as nutrients, temperature, water, as well as oxygen. However, when exposed to unfavourable situations like a lack of nutrients, an inadequate supply of oxygen or CO2, absence of water as well as moisture, some groups of bacteria possess the capability to make a defensive covering to guard themselves when exposed to severe environmental situations, allowing them to adapt comfortably to these situations. An endospore is a type of unique structure.
A non-vegetative structure formed by the Firmicute family of bacteria is an endospore. They have unique properties that allow them to live in harsh settings for extended periods of time.
These comprise of a dormant phase, a thick outer shell, as well as non-reproductive forms known as spores, which are resistant to high temperatures, radiation, and chemicals such as disinfectants as well as acids. This allows them to live, but also makes them tough to stain with simple dyes, therefore a special stain is used, which includes a specific dye as well as heat and steam. The Endospore stain, commonly referred to as the spore stain, is a type of staining technique. Its primary application is to locate and recognize the existence of bacterial endospores as well as vegetative forms in a cell. Clostridium spp. as well as Bacillus spp. are two examples of endospore-forming bacteria. These bacteria physically occur in soil, although they have important clinical repercussions because they cause human bacterial infections such as Clostridium tetani that causes tetanus, Clostridium botulinum, which causes paralysis, Bacillus cereus, which causes food poisoning, and Bacillus anthracis, which causes anthrax in cattle and humans.
Endospore staining procedures are categorised according to the chemicals employed.
- Malachite Green dye and safranin were employed in the Schaeffer Fulton Stain.
- Endospore staining by Dorner (Carbolfuchsin stain, acid alcohol, as well as Nigrosin solution)
Image Source Quizlet
Endospore staining objectives
- In order to find out the existence of an endospore.
- To recognise microorganisms that produce endospores
- To distinguish between vegetative and endospore forms
Endospore Staining Principle
Endospore staining is a differential stain which detects, identifies, and distinguishes an endospore from a vegetative cell (an underdeveloped endospore). The role’s basic function is to identify the presence or absence of endospores, although various techniques have improved the technique by increasing dye concentrations, extending heat fixation time, as well as using UV radiation.
With enhanced microscopy technology, some employ phase-contrast microscopy, which creates more precise morphologies of the bacterial endospore.
A. Stain by Schaeffer Fulton
The Schaeffer-Fulton technique is the most commonly used endospore staining technique in basic laboratories since it is straightforward as well as quick to detect the bacteria. It employs the use of Malachite green dye (an alkaline solution with a pH of 11-11.2 as well as a water-soluble dye) in conjunction with steamed-heat, which softens the endospore coating and allows dye penetration into the spore. The malachite green dye binds to the spore weakly and easily wipes away if rinsed with water without fixing, and that is why using steaming heat to permit the dye to enter the endospore is critical. To remove the malachite dye from the vegetative forms, water is used as a decolorizing agent. Finally, once the decolorizing agent has washed away the malachite dye, a counterstain, Safranin reagent, often known as the secondary stain, is employed to stain the vegetative forms of the undeveloped Furmigates (Water).
Malachite Green dye and Safranin work well in bacteria due to the positively charged alkaline nature of the Malachite Green reagents and the basophilic cytoplasm of the bacterial cell, which produces an attraction between the dye and the bacterial cell, making it easier to absorb the dye. The vegetative cell forms will appear under the microscope as pink-red stained particles that have taken up the counterstain, whilst the endospores will appear as green dotted particles (ellipses) which have taken up the Malachite green dye.
Reagents
- Malachite green pigment
- The element of water (Decoloriser)
- Safranin
Procedure
Materials used: Glass slide, Inoculation loop, and Bunsen burner
Microscope slide preparation (adapted for all other endospore staining techniques.)
- To remove any stains, clean the glass slide (with visible circles) with alcohol.
- Fill each circle with two tiny droplets of water using a sterile inoculation loop.
- Aseptically open the tube having the bacteria culture and burn it at the top prior to actually removing a loopful of the bacterial culture. Re-flame the tube after it has been closed.
- Put a drop of water on the bacterial culture on the slide.
- Leave to air dry completely.
- Heat-fix the slide with the smear facing up by passing it over the blue flame three or four times. IMPORTANT: Do not burn the bacteria-infested side.
- Leave to cool completely before attempting to stain.
Procedure for staining:
- To hide the streaks, use a piece of absorbent paper.
- Place the slide on a staining rack in front of a beaker/water bath filled with heating water.
- After being saturated with malachite green, let the absorbent paper to steam for 3-5 minutes.
- Set aside the stained absorbent paper for 1–2 minutes to cool before discarding it.
- Gently rinse the slide with tap water, turning it so that the water flows over the smeared discolouration. This is done to remove any surplus dye that may have been present on both sides of the slide, as well as any extra dye that may have coloured any vegetative forms in the heat-fixed smear.
- Add the counterstain, safranin, for 1 minute.
- Rinse the slide with water on both sides to remove the safranin reagent.
- Make sure the bottom of the slide is dry before viewing it with the oil immersion lens at 1000x for maximum magnification.
Endospore Staining Result
The vegetative forms will be stained pink/red by safranin, whereas the endospores will be stained green by malachite green dye.
Result interpretation
The vegetative forms stain pink/red since they consume the counterstain (Safranin), whereas the endospores acquire the green of Malachite green.
This is due to the fact that the malachite green enters the endospore with the help of heat from the steam during spreading as well as heat fixing, and the dye is not easily wiped away during the water–rinse.
Since the dye is easily wiped away by the thin outer layer of the vegetative forms, they pick up the last stain, which is the counterstain, and so appear pink-red.
B. The Dorner staining procedure
Reagents:
Stain with carbolfuchsin,
Decolorizing solution (acid-alcohol)
Contrast stain (Nigrosin solution)
Procedure
(The aforementioned process is used to prepare the microscopic slide.)
Procedure for staining
- Cover the smear with absorbent paper.
- Saturate it with carbol-fuschin and heat fix it for 5-10 minutes in a beaker or over a boiling water bath while adding more colour to the smear.
- Remove the absorbent paper and decolorize it with acid-alcohol for 1 minute; rinse with tap water and tap dry.
- Apply a thin layer of nigrosin reagent as a counterstain.
- Use a 1,000X oil immersion lens to look for endospores on the slide.
Result
Endospores are red, while vegetative cells are colourless.
Endospore staining applications
- To identify Firmicute groups of bacteria, such as Clostridium spp. as well as Bacillus spp.
- To recognize endospore-producing bacteria in samples.
- For distinguishing spore-producing bacteria from vegetative bacteria.
Endospore staining advantages
- As a Differential stain, it allows you to identify individual bacteria with endospores.
- It also detects and identifies the presence of vegetative forms in bacterial culture, as well as the presence of endospore generating bacteria.
Disadvantages
- It can simply detect the existence of endospore-forming bacteria.
NOTE
- The Klein method of endospore staining is a less widely used endospore staining technique.
- The usage of dyes differs between the Schahuffer Fulton and Klein staining processes; for example, in the Schahuffer Fulton stain, Malachite Green dye is utilised, whereas in the Klein methodology, Methylene blue solution is used.
Endospore staining Citations
- https://www.cartercenter.org/resources/pdfs/health/ephti/library/lecture_notes/env_occupational_health_students/MedicalBacteriology.pdf
- https://quizlet.com/85871953/stains-flash-cards/
- https://quizlet.com/192529190/microbiology-practical-review-flash-cards/
- https://en.wikipedia.org/wiki/Gram_stain
- https://en.m.wikipedia.org/wiki/Malachite_green
- https://ca.answers.yahoo.com/question/index?qid=20140530083604AAhVsS2
- https://brainly.com/question/10509484
- https://answers.yahoo.com/question/index?qid=20100704202926AA7Q6zs
- http://www.ruf.rice.edu/~bioslabs/bios318/staining.htm
- http://www.austincc.edu/microbugz/endospore_stain.php
- http://vlab.amrita.edu/?sub=3&brch=73&sim=208&cnt=2
- http://spot.pcc.edu/~jvolpe/b/bi234/lab/differentialTests/endospore_stain.htm
Related Posts
- Starch Hydrolysis Test : Objective, Principle, Procedure, Results
- Anisocytosis: Definition, Types, Causes, Symptoms, Treatment
- Endospore Staining: Principle, Procedure, Reagents, Results
- Flow Cytometry: Overview, Principle, Steps, Types, Uses
- Northern Blot: Overview, Principle, Procedure and Results
- MPV Blood Test: Calculation, High and Low MPV Value, Results
- Latex Agglutination Test: Objectives, Principle, Procedure, Results
- Iodine Test: Definition, Objective, Principle, Procedure, Results
- Eosin Methylene Blue (EMB) Agar
- Biuret Test for Protein: Purpose, Objectives, Principle, Procedure, Reagents
- Streak Plate Method: Meaning, Principle, Methods, Importance, Limitations
- Bile Solubility Test: Objective, Principle, Procedure, Results, Uses
- Butyrate Disk Test: Objective, Principle, Procedure, Results, Uses, Limitations
- Beta Lactamase Test: Objective, Principle, Procedure, Results, Limitations
- Bacitracin Susceptibility Test: Objective, Principle, Procedure, Results, Uses, Limitations