What is the Purpose of the Biuret Test?
Proteins are made up of hundreds of amino acids and are a complicated substance. Amino acids are amphoteric electrolytes that include carboxyl and amino groups, respectively, and act as an acid and a base. These ions are electrically neutral and do not migrate in the electric field since they have one positive charge and one negative charge. Dipeptide is formed when two amino acids are joined together by a peptide bond. With the elimination of a water molecule, a connection is established between the amino group of one amino acid and the carboxyl group of another amino acid. The condensation reaction is the process of forming a peptide bond. Similarly, a tripeptide is made up of three amino acids bonded by two peptide bonds, and as the chain lengthens, it is called a polypeptide. Biuret is a chemical created by heating urea to 1800 degrees Fahrenheit, resulting in the condensation of two urea molecules. Biuret is named for the peptide bonds in the reagent that yield a positive result for the test. It’s a broad test for substances with two or more peptide (CO-NH) links (proteins and peptides).
Biuret Test Objectives
- To determine the presence of a protein in a particular solution.
- To show that the peptide bond is present.
Image Created with BioRender.com
Biuret Test Principle (How the biuret Test works?)
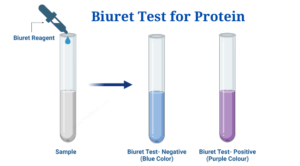
A Biuret test is a chemical test that determines whether or not a sample contains a peptide link. It is based on the biuret reaction, which produces a violet colour in an alkaline copper sulfate-treated peptide structure with at least two peptide links. Because the peptide has unshared electron pairs in nitrogen and oxygen of water, blue-colored copper II ions can form a complex with the peptide bonds in the presence of an alkaline solution. The Cu2+ ion, as well as the carbonyl oxygen (>C=O) and amide nitrogen (=NH) in the peptide bond, produce the colourful coordination complex. Once this complex has formed, the colour of the solution shifts from blue to purple. The more purple the tint, the more peptide-copper complexes there are. Any compound with at least two H2N-C, H2N-CH2-, H2N-CS-, or similar groups linked either directly or through a carbon or nitrogen atom will undergo the reaction. By co-ordinate bonds, one copper ion is likely coupled to six adjacent peptide connections. The colour intensity is proportional to the number of peptide bonds present in the responding protein molecule, as well as the number of protein molecules present in the reaction system.
A solution of sodium hydroxide (NaOH) or potassium hydroxide (KOH), hydrated copper (II) sulphate, and potassium sodium tartrate is used to make the Biuret reagent. The alkaline medium is made out of sodium hydroxide and potassium hydroxide, with potassium sodium tartrate added to chelate and thereby stabilise the cupric ions in the solution, or to keep their solubility in alkaline solution.
Requirements for Biuret test
- 1 % alanine, 5 % egg white (albumin)
- Biuret reagent
- Water bath
- Dry test tubes
- Pipettes
Biuret Reagents:
- Copper sulfate (CuSO40
- Sodium hydroxide (NaOH)
- Sodium potassium tartarate (commonly known as Rochelle salt)
Preparation Biuret reagent
- Biuret reagent is prepared by adding NaOH in CuSO4 solution, making it alkaline.
- To prepare 1000ml of Biuret reagent
- Take 1.5 gram of pentavalent copper sulphate (CuSO4) and 6 gram of Sodium potassium tartarate and dissolve them in 500 ml of distilled water
- **Sodium potassium tartarate is a chelating agent and it stabilize the copper ion
- Take 375 ml of 2 molar Sodium hydroxide
- Mix both the solution in volumetric flask and make it final volume to 1000 ml by adding distilled water.
Biuret Test Procedure
- Three clean and dry test tubes are required.
- Fill the test tubes with 1-2 mL of the test solution, egg albumin, and deionized water.
- Fill each test tube halfway with Biuret reagent (1-2 mL).
- Allow 5 minutes for the mixes to settle after a good shake.
- Keep an eye out for any hue shifts.
| Observation | Interpretation |
| There is no change in colour, i.e. the solution remains blue. | Proteins are not present (negative biuret test) |
| The colour of the solution changes from blue to deep purple. | Proteins can be found (positive biuret test) |
Click Here for Complete Biology Notes
Precautions
Presence of magnesium and ammonium ions interfere in biuret test. This can be overcome by using excess alkali.
Uses of the Biuret Test
- It can be used to determine how much protein is present in the urine.
- The biuret reaction with protein can be used to determine total protein quantitatively via spectrophotometric analysis.
Biuret Test Citations
- https://www.sciencedirect.com/science/article/pii/0009898175903514
- https://peptidesciences.com/information/peptide-bonds/
- https://onlinesciencenotes.com/biuret-test-principle-requirements-procedure-and-result-interpretation/
- https://quizlet.com/18722031/biology-chapter-5-flash-cards/
- https://microbiologyinfo.com/benedicts-test-principle-composition-preparation-procedure-and-result-interpretation/
- https://brainly.in/question/716138
- http://amrita.olabs.edu.in/?sub=79&brch=17&sim=205&cnt=1
- https://education.jlab.org/qa/charges_01.html
Related Posts
- Lineweaver–Burk Plot with Example
- Cell Wall: Definition, Diagram, Structure And Functions
- Phylum Porifera: Classification, Characteristics, Examples
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Epithelial Tissue Vs Connective Tissue: Definition, 16+ Differences, Examples
- 29+ Differences Between Arteries and Veins
- 31+ Differences Between DNA and RNA (DNA vs RNA)
- Eukaryotic Cells: Definition, Parts, Structure, Examples
- Centrifugal Force: Definition, Principle, Formula, Examples
- Asexual Vs Sexual Reproduction: Overview, 18+ Differences, Examples
- Glandular Epithelium: Location, Structure, Functions, Examples
- 25+ Differences between Invertebrates and Vertebrates
- Cilia and Flagella: Definition, Structure, Functions and Diagram
- P-value: Definition, Formula, Table and Calculation
- Nucleosome Model of Chromosome