Amino Acids Introduction
Amino acids are chemically as well as biochemically distinct from other natural chemicals because of their ampholytic capabilities.
- In a carboxylic acid, an amino group is located in the proximity to the carboxyl group, and it has a specific stereochemistry and structure.
- From 20 amino acids, proteins are biosynthesized, using a rigorous genetic control system. They are the basic building blocks of proteins.
- Because genes code for them, just 20 of the more than 300 amino acids occurring in nature may be used to make a protein. Amino acids that have been modified to be non-protein amino acids are also known as modified amino acids.
- The posttranslational modifications affect some residues, while some are amino acids that are found in living organisms and therefore do not form part of proteins.
Amino Acids Properties
A. Physical Properties
- Amino acids are crystalline solids that are colourless.
- There is a high melting point of 200o for all amino acids
- Their solubility ranges from water to alcohol to propanol and methanol, with the last being the most difficult to dissolve. When it comes to solubility, both the R-group of amino acids and the pH of the solvent are crucial.
- It’s decomposition when heated to a very high temperature.
- Optically active substances include all amino acids (except for glycine).
- Creation of a peptide bond: Peptide bonds among amino acids and their carboxylate groups are possible. When the alpha amino group of one amino acid bonds with an alpha-carboxyl group of some other amino acid, a -CO-NH-linkage is created. Peptide bonds are partially ionic and planar.
B. Chemical Properties
1 . The zwitterionic quality
It is made up of functional groups, at least including one that does indeed have a positive electrical charge and another negative. E ntire molecule has a total charge of zero. Many people are familiar with zwitterions from amino acids. A carboxylic group and an amine group are present (acidic). Due of its strength, the +NH2 group can accept H+ from the -COOH group, forming a Zwitterion. amino acids are usually found in solution as zwitterion (neutral).
2. Amphoteric Property
By virtue of the amine and carboxylic group present, amino acids are amphoteric.
3. Ninhydrin Test
A violet colour is formed when 1 ml of Ninhydrin solution is put to a 1 ml protein solution and heated.
4. Xanthoproteia Test
To detect aromatic amino acids (tyrosine, tryptophan, and phenylalanine) in protein solution, xanthoproteic testing is used. By reacting with Nitric Acid, benzoid radicals in the amino acid chain are converted into yellow compounds.
5. Sanger’s reagent reaction
A free amino group in the peptide chain reacts with Sanger’s reagent (1-fluoro-2, 4-dinitrobenzene) in a mild alkaline solution under cold conditions.
6. Reaction with nitrous acid
Because of this, Nitrous Acid interacts with the amino group to release Nitrogen.
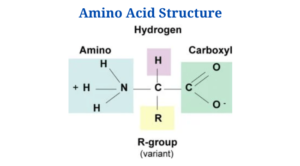
Amino Acids Structure
Every single one of the 20 amino acids is an alpha amino acid. You’ll notice that they’re bonded to the α -carbon with carboxyl groups as well as amino groups and side chains (R groups).
Exceptions are:
- That’s because it’s the only amino acid with no side chain. Two hydrogens are found in its -carbon.
- Nitrogen is part of a ring in proline.
- On the other end of the spectrum, each amino acid contains a characteristic side chain and an amine group. Amino acids have the same backbone, but their side chains differ.
- Asymmetric carbons are found in the -carbons of all 20 amino acids except glycine. Due to the absence of an asymmetric carbon atom, the glycine molecule is indeed not biologically active and is neither D nor L.
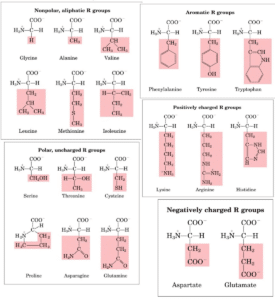
Classification of amino acids on the basis of R-group
- These amino acids have nonpolar and hydrophobic R groups, making them nonpolar and hydrophobic amino acids. Proline, Methionine, Glycine, Alanine and Valine.
- Tryptophan and tryptophan are nonpolar amino acids because of their aromatic side chains (hydrophobic). Hydrophobic interactions can involve anyone.
- As a result, they are much more water-soluble, or hydrophilic, than nonpolar amino acids since they possess functional groups which make hydrogen bonds with water. Asparagine, glutamine, cysteine, and serine are among the amino acids in this class.
- Acidic amino acids are those in which the R-group is acidic or negatively charged, as the name suggests. Amino Acids: Glutamic and Aspartic
- R-group amino acids that are positively charged. Histidine, Lysine, and Arginine
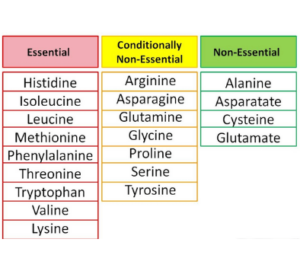
A. Nutritional categorization of amino acids
1 . Amino acids that are necessary (Nine)
For protein synthesis to proceed, nine amino acids must always be consumed in the diet.
This list includes histidine, isoleucine, leucine, lysine, methionine, phenylalanine, tryptophan, and valine.
2. Amino acids that are not essential to life (Eleven)
These amino acids can indeed be manufactured inside the body, thus they do not want to be obtained from food sources at all.
In addition to glutamine and arginine, other amino acids include tyrosine, cysteine and glycine as well as proline and serine.
3. Conditionally
B. Categorization of amino acids depending on metabolic fate
(a) Glucogenic amino acids: Amylucosides that cause weight gain In the process of gluconeogenesis, these amino acids act as precursors for the production of sugar. Proline, valine, methionine, cysteine, histidine and arginine are among the amino acids.
(b) Ketogenic amino acids: Amino acids that breakdown to create ketone bodies are known as ketogenic amino acids (KAAs). Leucine and Lysine are two essential amino acids.
(c) Both glucogenic and ketogenic amino acids: A combination of glucose-producing as well as ketogenic amino acids Such amino acids are broken down into ketone bodies and glucose precursors. Tyrosine and isoleucine are among the essential amino acids.
Functions of Amino Acids (Why Amino acids Are Important?)
- As peptides and proteins are the foundations of all living organisms, 20 extremely critical amino acids are necessary to sustain life.
- Polypeptide chains have a three-dimensional structure that is determined by the linear order of amino acid residues, and a protein’s function depends on its structure.
- To keep the body healthy, amino acids are essential. They promote: Hormone production, muscular construction, good nervous system function, and organ health.
- They are used by various tissues in the synthesis of proteins and nitrogen-containing substances (such as heme and creatine) or they are oxidised for energy production, depending on the type of amino acid.
- Nitrogen-containing substrates, such as amino acids, and carbon skeletons, result from the breakdown of food and tissue proteins.
- In the biosynthesis of neurotransmitters, hormones, porphyrins, and nonessential amino acids, nitrogen-containing substrates are employed.
- Citric acid cycle: The citric acid cycle uses carbon-skeletons as an energy source in the citric acid cycle.
Click Here for Complete Biology Notes
Amino Acids Citations
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Rodwell, V. W., Botham, K. M., Kennelly, P. J., Weil, P. A., & Bender, D. A. (2015). Harper’s illustrated biochemistry (30th ed.). New York, N.Y.: McGraw-Hill Education LLC.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.
- http://www-plb.ucdavis.edu/courses/bis/105/lectures/AminoAcids.pdf
- http://www.aqion.de/site/zwitterions
- http://www.aminoacidsguide.com/
Related Posts
- Lineweaver–Burk Plot with Example
- Cell Wall: Definition, Diagram, Structure And Functions
- Phylum Porifera: Classification, Characteristics, Examples
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Epithelial Tissue Vs Connective Tissue: Definition, 16+ Differences, Examples
- 29+ Differences Between Arteries and Veins
- 31+ Differences Between DNA and RNA (DNA vs RNA)
- Eukaryotic Cells: Definition, Parts, Structure, Examples
- Centrifugal Force: Definition, Principle, Formula, Examples
- Asexual Vs Sexual Reproduction: Overview, 18+ Differences, Examples
- Glandular Epithelium: Location, Structure, Functions, Examples
- 25+ Differences between Invertebrates and Vertebrates
- Cilia and Flagella: Definition, Structure, Functions and Diagram
- P-value: Definition, Formula, Table and Calculation
- Nucleosome Model of Chromosome