Aromatic Compounds
Aromatic compounds are unsaturated chemical compounds defined by the presence of one or more planar rings of atoms connected together by covalent bonds.
- The aromaticity of aromatic compounds is determined by the delocalization of shared electrons that result in their stability.
- The degree of aromaticity in different compounds varies, as it is determined by the bonding configurations of the atoms in the molecule.
- All aromatic compounds have a delocalized pi (π) cloud that covers all of the atoms in the aromatic ring uniformly. All aromatic compounds follow the Huckel’s ring, where n is an integer, hence the cloud must contain (4n+ 2) π
- A resonance effect takes place within a molecule when electrons are shared between distinct atoms. The stability of the molecules is due to the resonance effect.
- Resonance structures of single as well as double bonds are commonly used to illustrate the structure of these compounds, although the true structure has delocalized electrons shared by all of the atoms in the molecule.
- Despite the fact that these compounds are referred to as aromatic, not all aromatic compounds have a sweet odor, as well as not all aromatic compounds have a sweet odor.
- The most important group of aromatic chemicals is aromatic hydrocarbons. These are organic molecules with carbonas well as hydrogen atoms in them.
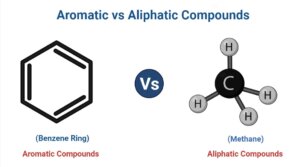
- The benzene ring, that is the simplest aromatic molecule, is the arrangement of carbon atoms in aromatic hydrocarbons.
- Aromatic compounds are nonpolar in general due to electron delocalization between atoms. Electrophiles can then attack the delocalized electrons to share the electrons, resulting in an electron-rich aromatic ring.
- Aromatic molecules are unsaturated as well as have a high resistance to addition reactions.
- The majority of these compounds have a flat planar structure as well as are cyclic compounds with a ring.
Aliphatic Compounds
Aliphatic compounds are a class of hydrocarbons with nonaromatic structures formed by single, double, or triple bonds between the atoms.
- The existence of an open-chain structure, that can be saturated or unsaturated, distinguishes aliphatic substances.
- Aliphatic hydrocarbons, that are made up of hydrogen as well as carbon atoms, are the most important type of aliphatic chemical. Other elements such as oxygen, nitrogen, chlorine, as well as sulfur may be present in these compounds.
- There are two types of aliphatic hydrocarbons based on the number of bonds between two carbon atoms. Open-chain hydrocarbons containing a single carbon-carbon link are known as saturated hydrocarbons.
- Unsaturated hydrocarbons, on the other hand, are open-chain hydrocarbons with double or triple carbon-atom bonds. Alkenes (double bonds) or alkynes are unsaturated hydrocarbons (triple bonds).
- Saturated hydrocarbons are more flammable as well as unstable than unsaturated hydrocarbons.
- Aliphatic compounds can be cyclic, but they don‘t obey Huckel’s rule as well as don’t exhibit aromaticity.
- Similarly, since aliphatic molecules lack a cyclic aromatic ring structure, they rarely exhibit resonance like aromatic compounds.
- Since of their high carbon content, the majority of known aliphatic compounds are combustible, allowing them to be used as fuels.
- Since of the slight difference in electronegativities between carbonas well as hydrogen, the bonds in aliphatic compounds are weakly polar.
- Due to the localisation of electrons in certain atoms, aliphatic molecules are involved in extra processes.
- Methane (CH4) is the most basic aliphatic chemical, from that all other aliphatic compounds are formed by polymerization or replacement.
Key Differences between Aromatic Compounds and Aliphatic Compounds
(Aromatic Compounds and Aliphatic Compounds)
[ninja_tables id=”5669″]
Aromatic compounds examples
Benzene
- Benzene is the most basic aromatic hydrocarbon, discovered by Faraday in 1823 but synthesized for the first time by Berthelot in 1870.
- It’s a six-carbon molecule with double bonds that alternate between the carbon atoms. It exists in the form of a ring structure that can be represented by various resonance configurations. C6H6 is the chemical formula for benzene.
- Benzene’s structure is cyclic, planar, as well as hexagonal, with the resonance structure being the most convincing.
- It behaves like a saturated compound in that it does not decolorize bromine solution or Baeyer’s reagent, despite the fact that it is an unsaturated compound.
- The delocalization of electrons within the ring as well as the exchange of double bonds between the carbon atoms give benzene this feature.
- Benzene as well as its derivatives are widely used in industry as well as can be manufactured or derived naturally from petroleum products.
Aliphatic compounds examples
Methane
- Methane is the most basic aliphatic hydrocarbon that exists in gaseous form in the atmosphere as well as is a by-product of various human activities.
- It’s a one-carbon compound with a single carbon atom linked to four hydrogen atoms. Methane has the chemical formula CH4.
- Methane is marginally soluble in water due to its weak polarity, although it has a specific gravity of 0.554, making it lighter than air.
- Methane is a very flammable gas with a mild, faintly bright flame when burned. It is a stable gas on its alone, but when mixed with air at a concentration of 5-14 percent by volume, it can be explosive.
- Methane, that is created as biogas for cooking, plays a significant role as a fossil fuel. It belongs to the greenhouse gas family as well as is one of the main contributors to global warming.
Aromatic Compounds and Aliphatic Compounds Citations
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 297, Methane” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Methane. Accessed 21 February, 2021.
- Gautam AD, Pant M and Adhikari NR (2017). Comprehensive Chemistry Part 2. Heritage Publishers and Distributors. Kathmandu, Nepal.
- https://pediaa.com/difference-between-aromatic-and-aliphatic-compounds/
- https://www.chegg.com/homework-help/questions-and-answers/q-organic-compounds-composed-carbon-hydrogen-oxygen-1-2-1-ratio-called-proteins-b-nucleoti-q10218997
- https://www.britannica.com/science/aromatic-compound
- https://www.britannica.com/science/aliphatic-compound
- https://vivadifferences.com/difference-between-aromatic-and-aliphatic-compounds/
- https://pediaa.com/difference-between-aliphatic-and-aromatic-hydrocarbons/
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example