Ionic Bond Definition
The ionic bond is a sort of chemical connection or linkage that takes place when oppositely charged ions or atoms with differing electronegativities attract one other via electrostatic attraction.
- One of the three basic forms of chemical bonds that develop between chemical units in order to achieve a stable state is the ionic bond.
- When an ionic bond is formed, electrons are transferred from one chemical unit to another. Charged ions are produced as a consequence
- Anions are negatively charged atoms that gain electrons as a consequence of the creation of ionic bonds. Cations are positively charged atoms that have lost electrons during the creation of ionic bonds.
- Metal ions act as cations most of the time, whereas nonmetal ions act as anions due to their electrical configuration.
- Electrostatic forces of attraction as well as repulsion between opposite charges as well as similar charges, respectively, generate ionic bonds. The final product of ionic bonding is known as an ionic compound.
- Ionic bonding are also seen in acid-base neutralisation reactions, as well as these molecules are commonly referred to as salts.
- Since every ionic compounds contains some degree of covalent bonding as a consequence of electron sharing, an ideal ionic bonding with total electron transfer from one chemical moiety to another does not exist.
- When the ionic character of the bond is larger than the covalent character, the term ionic bond is employed.
- The electrovalency of the atoms determines the strength of the ionic connection that is further determined by the atoms’ electronic structure.
What are electrovalent bonds, and how do they work?
- Electrovalent bonds are created when electrons from an electron-rich chemical unit are transferred to an electron-deficient chemical unit, resulting in charged ions. Ionic bonds are another name for electrovalent bonding.
Condition for Ionic Bonding
The Condition for the creation of ionic bonding between chemical compounds are as follows:
- The charges of the two elements or ions engaged in the creation of the ionic bond must be opposing. Metals as well as nonmetals are the most prevalent kinds of elements which form ionic bonding.
- One of the atoms (metal) participating in the bond formation must have a low ionisation or electron affinity, allowing it to easily shed electrons as well as create positively charged ions (cations).
- The next atom should have a high ionisation energy or electron affinity, allowing it to rapidly gain electrons as well as produce negatively charged ions (anions).
- The electronegativity difference between the two atoms engaged in the creation of an ionic bond should be more than 1.7.
- In order to be stable, the ionic molecule generated as a consequence of bonding must have high lattice energy.
- The bond’s ionic property should be greater than its covalent property.
Properties of ionic bonds
The features of ionic bonding as well as ionic compounds are as follows:
1 . State
- At room temperature, ionic compounds exist as solids since the strong electrostatic force of attraction holds the atoms together.
- Ionic compounds have a high lattice energy that makes them solid-state stable.
2. Solubility
- Most ionic chemicals are soluble in polar solvents such as water, but not in non-polar solvents such as benzene.
- The solubility is caused by the breaking of ionic bonds, that can only be accomplished by polar solvents such as water. The hydration of ions takes place as a result of the ionic dipole interaction, leading to solubility.
3. Boiling and melting point
- Since the ionic bond’s force of attraction is strong, ionic compounds have high boiling as well as melting points since breaking those bonds requires a lot of heat energy.
4. Density
- Because of the strong attraction between the chemical units engaged in ionic bonding, the ions can be orderly arranged in a close-packed crystal structure, and the crystal form of the chemicals increases the density.
5. Brittleness
- The brittleness of ions rises when they are arranged in a crystal lattice.
- Hammering these compounds causes repulsive forces to regenerate by bringing the ions closer together in the lattice. As a result of the repulsive forces, the compound becomes more brittle.
6. Non-directional
- Because of the force of attraction between oppositely charged ions works in all directions, ionic bonds are non-directional.
- Ionic bonds are non-directional and do not exhibit isomerism.
7. Electrical conductivity
- In the aqueous as well as molten states, ionic compounds disintegrated into charged ions. Electrical charges can be carried by charged ions, increasing electrical conductivity.
- Such compounds, however, can’t conduct electricity in the solid form since the ions are not free.
How are ionic bonds formed?
- Ionic bonds are made when the electronegativity difference between two atoms is 1.7 or greater.
- When such atoms get close enough, the difference in electronegativity creates an unequal sharing of electrons, with one atom losing an electron totally as well as the other accepting it.
- Ionic bonds are formed via a redox reaction in that a low-ionization-energy atom gives up one or more electrons in order to achieve a stable electron configuration. Cations are the chemical units that arise.
- To get a stable electron configuration, an atom of another element with a strong electron affinity accepts the electron from the other atom. Anions are the chemical species that result.
- When ionic bonds are created to produce the electronic configuration of noble gases in distinct blocks, the strength of the bonds is determined by the electrovalency of the atoms.
- As ionic compounds follow the rules of stoichiometry, exact ratios between anions as well as cations are seen during the formation of ionic bonds.
- Ionic bonds can only form if the total change in the energy of the system is favourable.
- The production of cations or the removal of electrons is an endothermic reaction that increases the system’s energy. During the ensuing attraction between the ions as well as the acceptance of electrons by the anions, however, the energy is reduced.
Ionic Bonds Examples
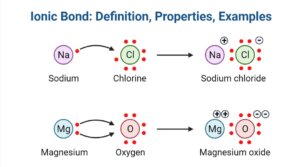
1. Ionic bonding in NaCl
- Ionic link between the metal ion, Na+, as well as the non-metal ion, Cl–, is created in NaCl.
- The sodium atom has the structure 1s2 2s2 2p6 3s1, suggesting that the outermost shell possesses a single valence electron.
- In order to establish a stable electronic state, the atom tends to shed one electron.
- Since chlorine is a non-metal, it has the electronic configuration 1s1 2s2 2p6 as well as prefers to accept one electron to achieve a stable electronic configuration.
- Sodium cation is formed, while the chlorine atom accepts one electron to form a chlorine anion.
2. Ionic bonding in MgO
- In the molecule MgO, the link between the magnesium atom as well as the oxygen atom is also an ionic bond since electrons are transferred from magnesium to oxygen.
- The outermost orbit of the magnesium atom possesses two electrons, resulting in low ionisation energy. To achieve a stable electrical state, the oxygen atom requires two electrons.
- Electrons can be transferred between the two atoms, resulting in the production of magnesium cation as well as oxygen anion.
Applications of Ionic Bonds
- Ionic substances such as NaCl as well as CaCO3 have a variety of uses in various fields. Since of the ionic connections, the compounds can exist in the solid state that increases their solubility as well as conductivity.
- Ionic stabilises the tertiary as well as quaternary structures of proteins, allowing them to maintain their form.
- Ionic bonds are also important in the development of chromosomal shapes, that hold the atoms of various organic molecules together.
- Ions in cells aid in the maintenance of cell potential as well as other vital tasks such as cell signaling as well as muscle contraction.
- Ionic bonds develop between organic substances, that assist the cell maintain its structure as well as execute numerous catalytic reactions as well as neuron activities.
Ionic Bond Citations
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd
- Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 2.2, Noncovalent Bonds. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21726/
- https://www.meritnation.com/ask-answer/question/give-reasons-1-in-the-formation-of-mgo-the-magnesium-atom-a/chemical-bonding/5454789
- https://www.icserankers.com/2020/11/icse-solutions-for-chapter2-chemical-bonding-class10-chemistry.html
- https://www.cliffsnotes.com/study-guides/chemistry/chemistry/chemical-bonding/ionic-bonds
- https://www.britannica.com/science/ionic-bond
- https://www.bbc.co.uk/bitesize/guides/ztc6w6f/revision/4
- https://socratic.org/questions/why-don-t-ionic-compounds-have-electrical-conductivity-as-a-solid-but-they-do-as
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example