Nitrogen Metabolism: The soil has limited amounts of nitrogen. Plants and microbes compete for this nitrogen. Therefore, it is a limiting nutrient for both agricultural and natural ecosystems. Let’s understand how this available nitrogen is cycled in the ecosystem a concept known as nitrogen metabolism.
Nitrogen Metabolism–
All the living organisms are basically composed of carbon, hydrogen, oxygen, nitrogen and many other forms of chemical elements. These elements contribute to finally organize various biomolecules present in a cell. Nitrogen is next to carbon in importance in living organisms. In a living cell, nitrogen is an important constituent of amino acids, proteins, enzymes, vitamins, alkaloids and some growth hormones. Therefore, study of nitrogen metabolism is absolutely essential because the entire life process is dependent on these nitrogen-containing molecules. In this lesson, you will learn about various aspects of nitrogen metabolism including nitrogen fixation and nitrogen assimilation in plants.
Nitrogen Cycle –
• Plants compete with microbe for limitation in Soil. So, N is a limiting nutrient (both natural & agricultural ecosystem).
• N2 has 2 N atoms joined by a triple covalent bond.
• Nitrogen fixation – Conversion of N2 to NH3.
• Lightning, UV radiation, industrial combustion, forest fire convert N to NO/NO2/N2O.
• Ammonification – Decomposition of organic N of dead plants/animals to NH3. Some NH3 volatile and re-enter the atmosphere but most get converted into nitrate by soil bacteria.
• 2NH3 + 3O2 → 2NO2- + 2H+ + 2H2O Nitrosomonas/ Nitrococcus.
• 2NO2- + O2 → 2NO3- (Nitrate) Nitrobacter
These steps are called Nitrification. These bacteria are chemoautotroph.
• It is absorbed by Plants → Leaves→ reduced to form NH3→ amine group of amino acid.
• Nitrate in Soil is reduced to nitrogen – Denitrification by Pseudomonas & Thiobacillus.
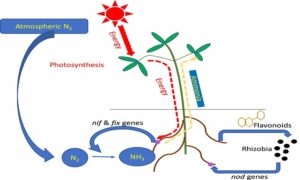
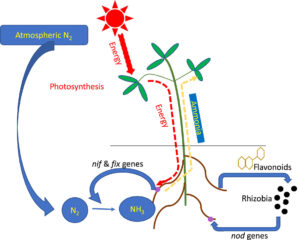
Biological Nitrogen Fixation-
• Only certain prokaryotic species are capable of fixing N.
• Reduction of N to NH3 by living organisms is biological N2 fixation.
• Nitrogenase for N reduction is present in prokaryotes N2 fixers.
• N2 fixing microbes could be free-living aerobic (Azotobacter, Beijernickia), anaerobic (Rhodospirillum), Bacillus, Anabaena, Nostoc or symbiotic.
Click Here for Complete Biology Notes
Symbiotic biological N2 fixation –
• Most prominent N2 fixing association is legume bacteria relation.
• Species of rod-shaped Rhizobium have a relationship with legumes’ roots such as alfalfa, sweet clover, sweet pea, lenticels, Garden pea, and clover bean.
• Common association on the root is nodules (small outgrowth).
• Frankia produce N2 fixing Nodule on non – leguminous plants (Alnus).
• Frankia & Rhizobium – free living in Soil but symbiont at N2 fixing.
• Nodules become pink due to leguminous haemoglobin or leghemoglobin.
Nodule formation –
• Rhizobia multiply & colonies surrounding of roots & attach to epidermal & root hair cell. Root hair curls & bacteria invade it.
• Infection thread is produced carrying bacteria into the cortex of root where they initiate Nodule formation in the cortex.
• Leads to differentiation of N2 fixing cells.
• Nodule formed establishes a direct vascular connection with the host for the exchange of nutrients. It contains nitrogenase & leghemoglobin.
• Nitrogenase is a Mo-Fe protein, catalyze the conversion of N2 to NH3 (first stable product).
N2 + 8e– + 8H+ + 16ATP —→ 2NH3 + H2 + 16ADP + 16Pi
• Nitrogenase is sensitive to molecule O2, requires anaerobic conditions.
• To protect EnzymeEnzyme, Nodule contains O2 scavenger – Leg haemoglobin.
• These live as aerobes under free-living conditions but anaerobe during N2 fixing.
• NH3 by nitrogenase require high input of energy. It is obtained from the respiration of host cells.
• Fate of Ammonia – At physiological pH, NH3 is protonated. NH4+ is quite toxic and cannot accumulate in plants.
• Reductive Amination- NH3 react with α-ketoglutaric to form glutaric acid.
• Transamination – Transfer of amino group from one amino acid to keto acid. The transaminase catalyzes these.
• Two essential amides – asparagine & glutamine
• Formed from two amino acids, aspartic acid & glutamic acid, by another amino group. The Hydroxyl part is another NH2- radicle. Amide contains more Nitrogen than Amino acid; they are transported to other components via xylem. In addition, along with transpiration, stream nodules export fixed nitrogen as ureides (Soyabean). These have high N: C
Related Posts
- Phylum Porifera: Classification, Characteristics, Examples
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Epithelial Tissue Vs Connective Tissue: Definition, 16+ Differences, Examples
- 29+ Differences Between Arteries and Veins
- 31+ Differences Between DNA and RNA (DNA vs RNA)
- Eukaryotic Cells: Definition, Parts, Structure, Examples
- Centrifugal Force: Definition, Principle, Formula, Examples
- Asexual Vs Sexual Reproduction: Overview, 18+ Differences, Examples
- Glandular Epithelium: Location, Structure, Functions, Examples
- 25+ Differences between Invertebrates and Vertebrates
- Lineweaver–Burk Plot
- Cilia and Flagella: Definition, Structure, Functions and Diagram
- P-value: Definition, Formula, Table and Calculation
- Nucleosome Model of Chromosome
- Northern Blot: Overview, Principle, Procedure and Results