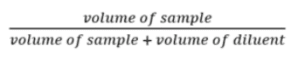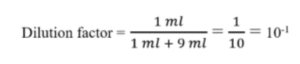Definition of Serial dilution
Serial dilution is a method of converting a dense solution into a more useable concentration by performing a series of repeated dilutions.
- Serial dilution, in layman’s words, is the process of diluting a solution with a dilution factor one step at a time.
- Serial dilution is commonly used in biology to reduce the concentration of cells in a culture to make the process easier.
Serial dilution’s Objectives
- The serial dilution method’s goal is to count the number of colonies cultured from successive dilutions of an unknown material to estimate its concentration (number of organisms, bacteria, viruses, or colonies).
- The density of cells is lowered in each step of serial dilution, making it easier to calculate the concentration of cells in the initial solution by computing the total dilution over the series.
- To avoid having to pipette very small quantities (1-10 l) to make a dilution of a solution, serial dilutions are often used.
- It is possible to create incubated culture plates with an easily countable number of colonies (about 30–100) and quantify the number of microorganisms contained in a sample by diluting it in a controlled manner.
Formulas and calculations for serial dilution
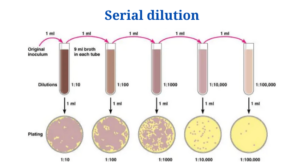
- Serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of standard quantities.
- Then, using a small measured volume of each dilution, a series of pour or spread plates are created.
- The extent of dilution is computed based on the expected concentration of cells/organisms in a sample.
- The dilution factor is raised, for example, if a water sample is taken from an excessively polluted location.
- A modest dilution factor, on the other hand, may be sufficient for a less contaminated sample.
- Serial two-fold and ten-fold dilutions are commonly used in the laboratory to titer antibodies and prepare diluted analytes.
- The dilution factor can be calculated for each individual test tube or as a cumulative dilution factor for the entire series in a serial dilution.
- Each tube in a set’s dilution factor is:
- 1 ml of sample is added to 9 ml of diluent for a ten-fold dilution. In this situation, the test tube’s dilution factor will be:
- Each tube after the first is a dilution of the previous dilution tube.
Now, for total dilution factor,
- Total dilution factor for the second tube = dilution of first tube × dilution of the second tube.
Example:
For the first tube, dilution factor = 10-1 (1 ml added to 9 ml)
For the second tube, dilution factor = 10-1 (1ml added to 9 ml)
Total dilution factor = previous dilution × dilution of next tube
= total dilution of 10-1 × 10-1 = 10-2
Calculator for Serial Dilution Online
- AAT Bioquest, Inc. (https://www.aatbio.com/tools/serial-dilution)
- Merck (https://www.sigmaaldrich.com/chemistry/stockroom-reagents/learning-center/technical-library/solution-dilution-calculator.html)
- Omni Calculator (https://www.omnicalculator.com/chemistry/serial-dilution)
- Endmemo (http://www.endmemo.com/bio/dilution.php)
- Handymath (https://handymath.com/cgi-bin/serdil6.cgi?submit=Entry)
- Tocris Bioscience (https://www.tocris.com/resources/dilution-calculator)
- Physiology Web (https://www.physiologyweb.com/calculators/dilution_calculator_mass_per_volume.html)
- Selleck Chemicals (https://www.selleckchem.com/dilutioncalculator.jsp)
Serial Dilution Procedure
The technique for a ten-fold dilution of a sample to a dilution factor of 10-6 is as follows:
- The sample/culture is placed in a test tube, followed by six test tubes containing 9 mL of sterile diluents (distilled water or 0.9 percent saline).
- It is necessary to use a sterile pipette.
- In the pipette, 1 ml of properly mixed sample/culture is drawn.
- After that, the sample is added to the first tube, resulting in a total amount of 10 ml. This yields a 10-1 initial dilution.
- The dilution is well blended by repeatedly emptying and filling the pipette.
- A fresh pipette tip is fitted to the pipette, and the old pipette tip is discarded.
- 1 ml of the mixture from the 10-1 dilution is now poured into the second tube. The overall dilution factor in the second tube is now 10-2.
- The remaining tube is then processed in the same way, with 1 ml from the previous tube added to the next 9 ml diluents.
- The final dilution for the bacteria/cells will be 10-6 due to the utilisation of six tubes (1 in 1,000,000).
Applications/Uses
- Serial dilution is used in biochemistry, pharmacology, physics, and homoeopathy, among other experimental fields.
- In microbiology, serial dilution is used to measure the concentration or quantity of cells/organisms in a sample in order to produce an incubated plate with a countable number of colonies.
- Serial dilution is a technique used in biochemistry to get the appropriate concentration of reagents and compounds from a greater concentration.
- Serial dilution is used in pharmaceutical laboratories to obtain the required concentration of chemicals and compounds since it is more effective than individual dilutions.
- Homeopathic dilutions occur when a material is diluted in distilled water or alcohol for use in homoeopathy. It is said that diluting a material boosts its potency by activating its vital energy.
Limitation/Problems of Serial Dilution
Although serial dilution is a valuable technique in laboratories, it is not without its drawbacks. Here are a few examples:
- During the propagation of the sample, an error may occur, and the transfer errors result in a less exact and precise transfer. The highest dilution has the most inaccuracies and the least accuracy as a result.
- Because serial dilution is done in a stepwise fashion, it takes a longer time to complete, limiting the method’s efficiency.
- Unlike other techniques like flow cytometry, serial dilution only allows for the reduction of bacteria/cells, not their separation.
- This procedure also necessitates the use of highly skilled microbiologists and aseptic technique experts.
Examples of Serial Dilution
- Tea or coffee are a simple illustration of serial dilution in our daily lives. In coffee, we use a set amount of cold press coffee and then pour water over it to achieve the necessary coffee concentration.
- In chemistry, dilution of acids and bases to attain a specified concentration is another example of serial dilution.
- Serial dilution of culture is also used to determine the amount of bacteria in a particular sample using a plating technique.
Click Here for Complete Biology Notes
Serial Dilution Citations
- Cullen, J. J., &MacIntyre, H. L. (2016). On the use of the serial dilution culture method to enumerate viable phytoplankton in natural communities of plankton subjected to ballast water treatment. Journal of applied phycology, 28(1), 279–298. https://doi.org/10.1007/s10811-015-0601-x
- https://www.biomol.com/dateien/Bethyl–Serial-Dilutions.pdf
- https://bio.libretexts.org/Bookshelves/Ancillary_Materials/Laboratory_Experiments/Microbiology_Labs/Microbiology_Labs_I/04%3A_Dilution_Worksheet_and_Problems
- https://www.biomol.com/dateien/Bethyl–Serial-Dilutions.pdf
- https://www.sciencedirect.com/science/article/pii/S0167701214002577
- https://www.coursehero.com/file/p71b91p/Serial-dilution-involves-taking-a-sample-and-diluting-it-through-a-series-of/
- https://www.wikihow.com/Do-Serial-Dilutions
- https://www.itwreagents.com/iberia/en/protein-biochemistry-and-electrophoresis
- https://quizlet.com/204569044/serial-dilutions-mastering-microbiology-lab-homework-flash-cards/
- https://quizlet.com/103092600/lab-quiz-2-flash-cards/
- https://en.wikipedia.org/wiki/Homeopathic_dilutions
- https://en.wikipedia.org/wiki/Colony-forming_unit
- http://dbpedia.org/page/Serial_dilution
- https://www.thoughtco.com/dilutions-from-stock-solutions-606085
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3941987/
- https://www.coursehero.com/file/16914203/Lab-Report-Dilutions/
- https://saveearthnow2015.wordpress.com/2015/06/28/analysis-of-drinking-water-contamination-2015/
Related Posts
- Phylum Porifera: Classification, Characteristics, Examples
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Epithelial Tissue Vs Connective Tissue: Definition, 16+ Differences, Examples
- 29+ Differences Between Arteries and Veins
- 31+ Differences Between DNA and RNA (DNA vs RNA)
- Eukaryotic Cells: Definition, Parts, Structure, Examples
- Centrifugal Force: Definition, Principle, Formula, Examples
- Asexual Vs Sexual Reproduction: Overview, 18+ Differences, Examples
- Glandular Epithelium: Location, Structure, Functions, Examples
- 25+ Differences between Invertebrates and Vertebrates
- Lineweaver–Burk Plot
- Cilia and Flagella: Definition, Structure, Functions and Diagram
- P-value: Definition, Formula, Table and Calculation
- Nucleosome Model of Chromosome
- Northern Blot: Overview, Principle, Procedure and Results