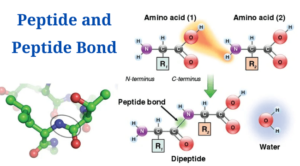
What is Peptide?
- A small chain of amino acids which, as soon as combined with some other peptides, produces a protein is a peptide.
- The quantity of amino acids inside a peptide may vary from two to fifty.
- Peptides are categorized into several groups depending on the amount of amino acids there; peptides having ten or less amino acids are called oligopeptides, whereas peptides having much more than ten amino acids are called polypeptides.
- Polypeptides containing roughly 100 amino acids are afterwards classified as proteins.
Peptide Bond Definition (What is Peptide Bond?)
- A sort of amide bond created among two molecules as soon as one molecule’s -carboxyl group combines with the -amino group of the other, making a water molecule.
- The peptide bond is indeed called as an isopeptide bond because it establishes an amide connection among the carboxyl group of one amino acid as well as the amino group of another amino acid at places other than the alpha.
- Creation of a peptide bond is an instance of a condensation reaction whichresults in dehydration (elimination of water).
- Covalent bonds which form when two amino acids combine to make a peptide chain are Peptide bonds.
- The amide bond has a partial double bond among its carbon as well as nitrogen, which stabilises the peptide bond.
- A resonance effect occurs when the nitrogen inside bond transports its single pair to the carbonyl group.
- Because electrons can indeed be delocalized over several atoms, the resonance structure is extremely stabilising.
- As a consequence of the partial double bond, the resonance structure not only stabilises the bond however also inhibits rotation around the amide bond.
- Peptide bonds contain such a planar structure which moves so little in the region of the C-N bond, whereas the other single bonds on each side of the C-N bond rotate very quickly.
Image Source: Wikipedia.
Peptide bond formation mechanism
- Mostly in synthesis of peptide bonds, a dehydration synthesis process is utilised.
- The carboxyl group of one amino acid moves towards the amino group of another amino acid during the creation of a peptide bond.
- The carboxyl group of the first amino acid is then stripped of one hydrogen and one oxygen atom (COOH). In contrast, one hydrogen is removed from the other amino acid’s amino group (NH2).
- As a result, the production of a water molecule (H2O) and the creation of an amide bond (C-N) among the two amino acids.
- Creation of a peptide bond among two amino acids leads in the production of a dipeptide molecule.
- Once the carboxyl group of one amino acid condenses with the amino group of another amino acid, a peptide bond is created, generating a water molecule.
- In living beings, the creation of the peptide bond is an endergonic reaction which needs energy, which is generated from ATP.
- This process is known as a dehydration synthesis reaction since it comprises the elimination of a water molecule.
Mechanism of Peptide bond degradation
- The peptide link is degraded via hydrolysis, which necessitates the existence of water molecules.
- Because the amide bond among the amino acids is maintained by the partial double bond, the degradation reaction is indeed very slow.
- Carbon atom has a small positive charge because of the partial double bond among the carbon as well as nitrogen molecules.
- When there is water present, the OH– ions of water target the carbon atom, causing the peptide bond to degrade.
- The water’s leftover hydrogen ion subsequently targets the nitrogen atom, leading to the amino group.
- As a consequence, the peptide molecule is divided into two units: one with a carboxyl group as well as one with an amino group.
- Peptide degradation is an exergonic reaction which produces 8-16 Kjol/mole of energy.
- Protein breakdown procedures are typically conducted by proteolytic enzymes such as proteases as well as peptidases since they are relatively sluggish.
Hydrolysis of Peptide bonds
- The foremost step in all protein hydrolysis reactions is peptide bond hydrolysis.
- One of the most frequent type of protein breakdown is acid–catalyzed peptide bond hydrolysis.
- Peptide hydrolysis is indeed required in several synthetic reactions in which amino acids from one peptide are split as well as transported to another peptide, leading in the synthesis of distinct peptides.
- Likewise, many peptides as well as proteins collect in cells, causing toxicity. Peptide bond hydrolysis is also required for the elimination of these poisons.
- Peptide bond hydrolysis is indeed an essential phase in protein digestion in living organisms.
- Peptide bond hydrolysis happens in the existence of water and is catalysed by the existence of acid.
- Peptide bond hydrolysis is one of the peptide bond degradation methods in which polypeptides are either broken down into small peptides or small peptides are broken into individual amino acids.
Examples
- The peptide bond is found in all proteins and is responsible for binding the amino acids in the chain together.
A monopeptide is a peptide which includes only one amino acid.
A dipeptide is a peptide which includes two amino acids.
Tripeptide: a peptide composed of three amino acids.
Tetrapeptide: a peptide with four amino acids.
Five amino acids make up a pentapeptide.
Six amino acids make to a hexapeptide.
Heptapeptide: a peptide with seven amino acids.
Eight amino acids make to an octapeptide.
Questions and Answers for Revision (FAQ)
What exactly is a peptide bond?
A sort of amide bond created among two molecules after one molecule’s -carboxyl group combines with the -amino group of another, producing a water molecule is a peptide bond.
What amino acid components are required in a peptide bond?
A peptide bond is formed among the carboxyl group of one amino acid as well as the amino group of another amino acid.
How do you recognise a peptide bond?
A peptide bond can be identified using the biuret test.
Is it possible for a peptide bond to be covalent?
A peptide bond is, indeed, a covalent bond.
Peptide Bond Citations
- https://www.peptidesciences.com/information/peptide-bonds/
- https://quizlet.com/164305942/study-flash-cards/
- https://quizlet.com/116549295/chapter-4-bio-302-flash-cards/
- https://www.sciencedaily.com/terms/peptide_bond.htm
- https://www.ncbi.nlm.nih.gov/books/NBK21750/
- https://encyclopedia2.thefreedictionary.com/Polypeptide+chains
- https://answersdrive.com/what-is-a-chain-of-peptides-1326184
- https://www.researchgate.net/publication/6976681_Resonance_Structures_of_the_Amide_Bond_The_Advantages_of_Planarity
- https://www.researchgate.net/publication/6835267_Opinion_-_Do_we_underestimate_the_importance_of_water_in_cell_biology
- https://www.chegg.com/homework-help/questions-and-answers/1-bond-carbon-nitrogen-considered-polar-covalent-atom-c-n-bond-develop-partial-negative-ch-q34037077
- https://chem.libretexts.org/Courses/University_of_Illinois%2C_Springfield/UIS%3A_CHE_124_(Morsch_and_Andrews)/Book%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)/18%3A_Amino_Acids%2C_Proteins%2C_and_Enzymes/18.03_Peptides
Related Posts
- Phylum Porifera: Classification, Characteristics, Examples
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Epithelial Tissue Vs Connective Tissue: Definition, 16+ Differences, Examples
- 29+ Differences Between Arteries and Veins
- 31+ Differences Between DNA and RNA (DNA vs RNA)
- Eukaryotic Cells: Definition, Parts, Structure, Examples
- Centrifugal Force: Definition, Principle, Formula, Examples
- Asexual Vs Sexual Reproduction: Overview, 18+ Differences, Examples
- Glandular Epithelium: Location, Structure, Functions, Examples
- 25+ Differences between Invertebrates and Vertebrates
- Lineweaver–Burk Plot
- Cilia and Flagella: Definition, Structure, Functions and Diagram
- P-value: Definition, Formula, Table and Calculation
- Nucleosome Model of Chromosome
- Northern Blot: Overview, Principle, Procedure and Results