Definition of Aldoses
A monosaccharide with a carbon backbone as well as a carbonyl group at carbon-1 is known as an aldose.
- Aldoses have the same general formula as other carbohydrates, Cn(H2O)n. Each hydroxyl group is linked to a carbon atom in the carbon backbone.
- Since aldoses have an asymmetrical carbon core, they all display stereoisomerism. Depending on the chirality of the asymmetric carbon, these molecules can exist in either L-form or D-form.
- D-aldoses are aldoses with alcohol groups on the right, whereas L–aldoses are aldoses with alcohol groups on the left.
- Aldoses are polyhydroxy aldehydes that can also take the form of hemiacetals, that have a cyclic ring structure. Carbohydrates containing more than four carbon atoms have a cyclic structure.
- Since many biological systems can only employ one enantiomer of a carbohydrate, aldoses are often given names that reflect their stereoisomerism.
- Aldoses can also tautomerize into ketoses via a dynamic process involving the production of an enol intermediate. Tautomerization can be reversed, as well as the aldo-form is typically more stable than the enol-form.
Definition of Ketosis
A ketose is a monosaccharide that contains a carbon backbone as well as a carbonyl group inside it.
- The general formula for ketoses is RCOR’, where R is an alkyl group that may or may not be the same as the other R’.
- Since monosaccharide ketoses may be tautomerized into aldehyde, that is subsequently oxidised, they are all reducing sugars. Ketones attached to glycosides, on the other hand, are nonreducing sugars.
- Ketones lack a carbonyl group at the end of the chain, resulting in a hemiketal cyclic ring structure, as opposed to the hemiacetal ring structure found in aldehydes.
- In ketoses, the carbon atoms are asymmetrical, resulting in various sugar forms due to the chirality of the asymmetrical carbon. The position of the hydroxyl group on the carbon backbone distinguishes the L- as well as D- types of ketoses.
- Seliwanoff’s test can distinguish ketoses from aldoses. The test is predicated on a faster dehydration reaction in ketosis, that results in a faster test result.
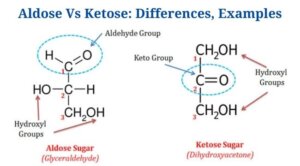
Key Differences between Aldose and Ketose
(Aldose Vs Ketose)
[ninja_tables id=”5481″]
Aldose examples
Glucose
- Glucose is an aldose monosaccharide sugar with the chemical formula C6H12O6 that is produced largely by photosynthesis in plants as well as algae.
- Since glucose can be used by a wide range of organisms, it is the most significant source of energy for many of them.
- It can also be stored as a polymer in plants, such as starch, as well as in animals, such as glycogen. During metabolism, the polymers are broken down into glucose units.
- D-glucose is the naturally occurring type of glucose, whereas L-glucose can be synthesised for specialised use.
- D-glucose is more significant than L-glucose since biological systems have D-glucose use pathways.
- Glucose has six carbon atoms as well as an aldehyde group, making it an aldohexose. The glucose molecule can be found in two forms: open-chain (acyclic) as well as ring (cyclic).
Ketose examples
Fructose
- Fructose, along with glucose as well as galactose, is a simple hexose sugar present in plants as well as is one of the three dietary monosaccharides.
- Fructose has a ketone functional group, as well as fructose’s ring structure forms at the second carbon position. Fructose has a 5-carbon ring structure with an intramolecular hemiacetal structure.
- It’s the most water-soluble of all the sugars, as well as it forms a sucrose-like disaccharide structure with glucose.
- High-fructose corn syrup is made with glucose monosaccharides taken from plant sources such as sugar cane, maize, as well as beets.
- Internal hydrogen-bonding stabilises the hemiketal structure of fructose, resulting in the crystalline form. D-fructopyranose is the crystalline form.
Aldose Vs Ketose Citations
- https://quizlet.com/49073732/carbon-chemistry-flash-cards/
- https://en.wikipedia.org/wiki/Ketose
- https://en.m.wikipedia.org/wiki/Ketone
- https://en.m.wikipedia.org/wiki/Aldose
- https://diabetestalk.net/blood-sugar/how-many-chiral-centers-are-there-in-the-open-chain-form-of-glucose-in-the-cyclic-form
- https://diabetestalk.net/blood-sugar/glucose-structure
- https://byjus.com/jee/fructose-structure/
- https://byjus.com/chemistry/structure-of-glucose-and-fructose/
- https://www.sciencedirect.com/topics/chemistry/glyceraldehyde
- https://www.ansaroo.com/question/why-is-d-glucose-more-common-than-l-glucose
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example