Definition of Ester
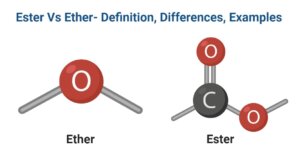
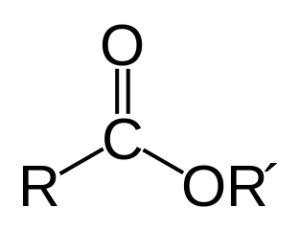
Fig: Ester General Structure
- Esters have the chemical formula RCOOR’, with R as well as R’ being the same or distinct organic groups.
- Esterification is the interaction between carboxylic acids as well as alcohols in the presence of acid that results in the creation of esters.
- Esters have a carbonyl group that is joined to an oxygen atom to produce a functional group. The IUPAC names esters with the suffix “-ate.”
- Esters feature 120° C-C-O as well as O-C–O angles due to the presence of a carbonyl core, as well as their functional groups are flexible.
- The flexibility of the angles allows for certain physical properties of the compounds, such as a lower melting temperature as well as enhanced volatility.
- The inclusion of a carbonyl group increases the polarity of the compound since the charge created can be dispersed between the carbon as well as oxygen to form a stable structure.
- Esters are mostly utilized in the food industry to incorporate the aroma of a variety of fruits, such as apples, bananas, as well as pears.
- Trimesters, that are vital for the structure as well as energy of living systems, can also be found in fats.
Definition of Ether
Fig: Ether General Structure
Ether is a class of chemical compounds made up of two alkyl or aryl groups joined by the ether group -O-
- Ether has the chemical formula R-O-R’.
- Based on the sort of alkyl or aryl groups present on the sides of the oxygen atom, ethers can be classed as symmetrical, mixed, or asymmetrical.
- A simple or symmetrical ether is one in that the alkyl or aryl groups are the same, but mixed or asymmetrical ethers are those in that the groups are different.
- In ethers, the C-O linkage is bent, usually at an angle of 111°, as well as the bond is flexible enough to rotate.
- Since the oxygen atom is more electronegative than the carbon atom, ethers are more acidic than simple hydrocarbons as well as esters. Ethers are designated by the general formula ‘alkoxy alkane‘ in the IUPAC naming system.
- Ethers have a lower boiling point as well as are less soluble than their alcohol counterparts. These come in the shape of a colorless, pleasant-smelling liquid.
- Diverse ethers have different industrial applications, since some are required in medicine as well as pharmacology for the synthesis of anesthetics.
- Ethyl ether, in particular, is a great solvent for extracting a variety of compounds.
- Ethers’ ability to be employed as solvents due to the likelihood of hydrogen bonds forming with other molecules.
- Dehydration of alcohols as well as alkyl halides can be used to make ethers artificially. The Williamson ether synthesis is the most often used method of ether synthesis.
Key Differences between Ester and Ether
(Ester Vs Ether)
[ninja_tables id=”5487″]
Esters Examples
Ethyl Acetate
- Ethyl acetate, sometimes known as ethyl ethanoate, is a molecular ester having the formula CH3-COO-CH2-CH3.
- Ethyl acetate is produced through esterification in the presence of an acid from acetic acid and ethanol.
- Because of its inexpensive cost, low toxicity, and fruity odour, it is largely employed as a solvent. It is employed as a solvent in the manufacture of pharmaceuticals and other pharmaceutical goods.
- Ethyl acetate is also found in wine as a byproduct of the fermentation of volatile organic acid and ethyl alcohol.
- Furthermore, it has applications in laboratories for column chromatography and chemical extractions.
- Ethyl acetate can also be used as an asphyxiant in entomology during insect collection and research.
Ether Examples
Diethyl ether
- Diethyl ether is an organic molecule with the chemical formula (C2H5)2 It is made up of two carbon atoms.
- It’s a colourless, transparent liquid with an anaestheticodour. It has a lower density than water as well as is water soluble.
- Since the oxygen atom is connected to two ethyl groups, diethyl ether is a symmetrical ether.
- The hydration of ethylene produces diethyl ether in both laboratories as well as businesses.
- It is a highly flammable liquid that is used to produce carbon dioxide as well as water through combustion.
- It has a high cetane number as well as is used to make petroleum distillates as well as gasoline as a starting fluid.
Ester Vs Ether Citations
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 8857, Ethyl acetate” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Ethyl-acetate. Accessed 18 March, 2021.
- https://www.scribd.com/presentation/360055436/Mineral-Compounds-Autosaved
- https://www.coursehero.com/file/12238622/prelab-6/
- https://www.scienceabc.com/pure-sciences/what-are-esters-formation-properties-and-uses.html
- https://www.chemguide.co.uk/organicprops/alcohols/esterification.html
- https://www.britannica.com/science/ester-chemical-compound
- https://kylefoster.me/acetato-de-etilo-msds-67/
- https://en.wikipedia.org/wiki/Ether
- https://en.wikipedia.org/wiki/Diethyl_ether
- https://en.unionpedia.org/Kilogram
- https://courses.lumenlearning.com/trident-boundless-chemistry/chapter/functional-group-names-properties-and-reactions/
- https://courses.lumenlearning.com/introchem/chapter/esters/
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example