Covalent Bond Definition
The covalent bond is a sort of chemical connection formed by the mutual sharing of pairs of electrons between atoms of the same or different elements.
- Attractive forces among electrons as well as nuclei, and repulsive forces between shared electrons, result in covalent bonding.
- In a covalent link, two atoms share the same number of electrons, that is referred to as the atoms’ covalency.
- Atoms involved in covalent bonding, like all other types of bonding, share electrons to establish a stable electronic configuration.
- Although electrostatic forces are the most common form of covalent bonding, additional interactions such as -bonding, -bonding, bending binds, three-center two-electron bonds, as well as three-center four-electron bonds are also present.
- When the total energy of the produced compounds is less than the total energy of the separated atoms, covalent bonds occur.
- In 1916, American chemist G. N. Lewis proposed the concept of covalent bonding.
- Covalent bonding is significantly more common than other kinds of bonding, as well as it can be found in compounds containing other kinds of bonding as well.
- Covalent bonding, unlike other types of bonding, can occur between two atoms of the same element, resulting in the element’s molecule form.
- In some interactions, a type of covalent bond known as coordinate covalent can be seen where the shared pair of electrons between two atoms comes from a single atom.
- The production of hundreds of organic compounds found in living beings is made possible by covalent bonding between carbon atoms, that is the basis of organic chemistry.
- When electrons are shared by more than two atoms in organic compounds, the electrons are said to be localised in order to establish a stable structure.
Covalent Bonding Conditions
The requirements for the creation of covalent bonding between chemical compounds are as follows:
- The most significant criteria for forming a covalent bond is that the energy of the new complex is less than the energy of the separated atoms.
- True covalent bonds are formed between atoms with identical electronegativities, as well as the two atoms involved in the creation of the covalent bond must be electronegative.
- The atoms must have a high ionisation energy so that electrons from their outermost orbit are not easily lost.
- The elements engaged in the creation of the covalent bond must have more than 4 electrons in their outermost orbit in order to achieve a stable configuration via sharing electron pairs.
Properties of covalent bonds
The properties of covalent bonding as well as covalent compounds are as follows:
1 . State
- Depending on the nature as well as amount of covalent bonds between atoms, covalent substances can exist in all three states of matter.
- Covalent compounds in the gaseous state are primarily held together by mild electrostatic forces, but those in the solid form are held together by stronger forces.
- H2O in a liquid state, CO2 in a gaseous state, as well as diamond in a solid state are all instances of covalent compounds in distinct states.
2. Boiling and melting point
- Because the boiling as well as melting points of covalent compounds are determined by the degree of attraction between the atoms or the strength of the bond, they are lower than those of ionic compounds.
3. Electrical conductivity
- Except for graphite, that is a good conductor of electricity, covalent compounds can’t conduct electricity under normal conditions.
- Covalent chemicals cannot transmit electricity in an aqueous or molten state because they do not produce ions.
4. Directional nature
- Covalent bonds are directional because some orbitals which are oriented towards a specific axis overlap.
- Strength of the bond is determined by the overlapping of the orbitals, therefore efficient overlapping results in stronger bonds.
- The direction of the created bond is determined by the orbitals involved in its formation.
- The s-orbitals are symmetrical as well as can create covalent bonds in any direction, whereas the p-orbitals are oriented along a certain axis as well as establish stable bonds in that direction.
- Because of the directed character of the bonds, different covalent compounds exhibit isomerism.
5. Solubility
- Non-polar solvents dissolve covalent molecules, that do not dissolve in polar solvents.
- Because of intermolecular hydrogen bonding with water molecules, some covalent compounds may dissolve in water.
- The lack of polarity of the bond causes insolubility of covalent compounds in polar liquids.
Types of covalent bonds
Covalent bonds come in a variety of shapes as well as sizes
Depending on the quantity of covalent bonds in a chemical, multiple types of covalent bonds exist. The number of covalent bonds is also determined by how many electrons are shared.
1 . Single Covalent Bond
- Single covalent bonds are created when the interacting atoms share a single pair of electrons. The symbol for a single covalent bond is (-).
- Compounds with single covalent bonds have a lower density, as well as the bonds are weaker but more stable than those with multiple covalent bonds.
- In single covalent bondsthe length of the bond is the longest because two electrons hold the nucleus with less force.
- Single covalent bonds can be found in elements that are widely apart in the periodic table.
- The covalent bond between hydrogen as well as chlorine atoms in HCl is an example of a single covalent bond.
2. Double Covalent Bond
- Double covalent bonds are created when two pairs of electrons are shared by the atoms involved. The symbol (=) denotes a double covalent bond.
- A double covalent bond has a strength that is halfway between single as well as triple covalent bonds. In the same way, the bond length is shorter than a single covalent bond yet longer than a triple covalent link.
- A double covalent bond arises in elements having four or more electrons in their outermost orbit that are closer on the periodic table.
- Because the shared electrons have a higher reactivity, double bonds are frequently less stable than single bonds.
- The link between carbon as well as oxygen atoms in CO2 is an example of a double covalent bond.
3. Triple Covalent Bond
- A triple covalent bond is created when three pairs of electrons are shared by the atoms involved. (≡) represents a triple covalent bond.
- Triple covalent bonds are the strongest covalent bonds because three pairs of electrons hold the nuclei together. Because larger forces draw the links closer together, the bond length is the shortest.
- The triple covalent bond is the least stable because the increased electron density causes the bond to be more reactive.
- This form of connection happens between atoms of the same element or elements in the periodic table that are relatively near together.
- The bond between two nitrogen atoms in the N2 molecule is an example of a triple covalent bond.
What causes covalent bonds to form?
- Covalent bonds develop in compounds in that none of the atoms engaged are metal atoms with low enough ionisation energies to allow electron loss.
- As a consequence, the atoms in the compounds tend to share electrons in order to achieve a stable electronic configuration for both.
- Covalency is more common in elements on the periodic table’s right side (mostly nonmetals).
- Lewis words can be used to illustrate the concept as well as development of a covalent bond. A covalent bond is a shared pair of electrons, according to Lewis.
- The lowering of the energy of the atoms engaged is the most significant condition for the creation of the covalent bond.
- When electrons are shared, the energy is lower than when they are attracted by a single nucleus because the electrons are shared between two attractive nuclei.
- Both atoms achieve a stable noble gas form with decreased energy as a result of sharing electrons.
- In a covalent bond, the shared pair of electrons is referred to as a bonding pair, while the rest of the electrons on the outermost orbit (if present) are referred to as lone pairs.
- Two atomic nuclei of the participating atoms attract the bonding pairs of electrons (one or more based on the number of bonds) so that they can be shared among the atoms.
- When the electronegativities of two atoms are too small for electron transfer to take place, a covalent bond takes place.
- The bonding electrons in covalent compounds are the glue that holds the atoms of the molecules together.
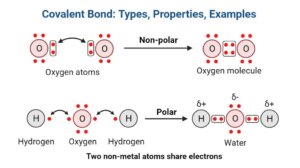
What are polar covalent bonds?
- A polar covalent bond is one in that the common pair of electrons is distributed unequally between the two atoms.
- Because one of the atoms gets slightly positively charged as well as the other becomes slightly negatively charged, polar covalent bonding produces a tiny electrical dipole moment.
- The atoms’ charges are less than a whole unit charge, that is represented by delta plus (δ +) as well as delta negative (δ -).
- Polar covalent bonds are a combination of the covalent as well as ionic bonds. When the electronegativities of the atoms involved differ, the majority of covalent bonds are polar bonds.
- The covalent link between hydrogen as well as oxygen in a water molecule is an example of a polar covalent bond.
- Polar covalent bonds allow molecules to interact with other molecules that have a dipole moment.
What are Non-polar covalent bonds?
- A non-polar covalent bond is one in that the shared pair of electrons is spread evenly amongst the two participating atoms.
- In a non-polar covalent connection, the two atoms share the same number of electrons.
- Non-polar covalent bonds are frequently formed between two non-metal atoms that have minimal electronegativity or between distinct atoms with negligible electronegativity.
- Non-polar covalent bonding can form from polar covalent bonds with bonding electrons arranged in a specific pattern in some situations.
- Non-polar covalent bonding is the ideal covalent bonding that produces molecules with features that distinguish them from polar compounds.
What are coordinate covalent bonds?
- A coordinate covalent bond is a chemical bond between two atoms in that a donor atom donates a lone pair of electrons as well as an acceptor atom accepts them.
- A dative bond is another name for this type of relationship.
- A coordinate covalent bond is a sort of covalent bond in that the bond is created by the sharing of electrons, but only by a single atom.
- A coordinate covalent bond oriented at the acceptor atom is denoted by the symbol (→).
- A coordinate covalent bond is a sort of covalent bond that forms between metal ions as well as ligands as well as has two centresas well as two electrons.
- The bond between the nitrogen as well as hydrogen atoms in NH4+ is an example of a coordinate covalent bond.
Covalent Bonds Examples
1 . Covalent bonding in HCl
- In HCl, a hydrogen atom forms a covalent link with a chlorine atom. Because the electronegativities of the two atoms differ, the covalent link in HCl is a polar covalent bond.
- Despite the fact that hydrogen as well as chlorine are farther apart in the periodic table, their electronegativities are too small to be ionic.
- Each of the atoms involved shares one element, allowing hydrogen as well as chlorine to achieve a stable electronic state.
- The hydrogen as well as chlorine atoms gain a little positive as well as negative charge, respectively, due to the polar covalent link.
2. Covalent bonding in O2
- Covalent bonding between atoms of the same element can also result in the molecular form.
- The two oxygen atoms have a double covalent link in that each atom shares a pair of electrons.
- Because no difference is there in electronegativity between the two atoms, the covalent bond in O2 is a nonpolar covalent bond, allowing mutual electron sharing.
- Because the non-polar covalent bond is weaker than the polar covalent bond, the oxygen molecules generated are gaseous.
Covalent Bonds Applications.
- The plant contains a variety of covalent compounds which are employed for various purposes. Covalent substances are made up of covalent bonds as well as range from gases like O2, N2, as well as CO2 to liquid acids like HCl as well as H2SO4.
- The production of hundreds of organic compounds which are vital for the structure as well as function of living beings is one of the most important uses of covalent bonding.
- Covalent connections facilitate the development of macromolecules, such as glycosidic links between sugar molecules as well as peptide bonds between amino acids.
- Hydrogen bonding is another type of covalent bonding that allows distinct bond characteristics to be exploited for various reasons.
Covalent Bonds Citations
- Robert J. Ouellette, J. David Rawn. Structure of Organic Compounds. Principles of Organic Chemistry, Elsevier. 2015. Pages 1-32. https://doi.org/10.1016/B978-0-12-802444-7.00001-X.
- Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 2.1, Covalent Bonds. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21595/
- https://www.technologyuk.net/science/matter/chemical-bonding.shtml
- https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/covalent-bond
- https://www.quora.com/Why-is-H2O-a-covalent-bond
- https://study.com/academy/lesson/polar-and-nonpolar-covalent-bonds-definitions-and-examples.html
- https://gradeup.co/jee-main-short-notes-chemical-bonding-i-80e43510-e861-11e6-b04c-1c9be0d6cce8
- https://byjus.com/jee/covalent-bond/
- https://www2.estrellamountain.edu/faculty/farabee/BIOBK/BioBookCHEM1.html
- https://www.yourdictionary.com/covalent-bond
- https://www.thoughtco.com/definition-of-dative-bond-604985
Related Posts
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Saturated vs Unsaturated Hydrocarbons: Definition, Differences, Examples
- Ethanol Vs Methanol: Definition and 10+ Differences
- Hydrogen Bond: Properties, Definition, Types, Examples
- Nitrate Vs Nitrite: Definition, Differences, Examples
- Aromatic Compounds vs Aliphatic Compounds: Definition, Differences, Examples
- Compound Vs Mixture: Definition, Differences, Examples
- Elements Vs Compounds: Definition, Differences, Examples
- Molecules Vs Compounds: Definition, Differences, Examples
- Hard water Vs Soft water: Definition, Differences, Examples
- Glucose Vs Sucrose: Definition and Key Differences
- 13+ Difference Between Atom and Molecule with Examples
- How to Balance Chemical Equation: Methods, Steps, Examples
- Ionic Bond: Definition, Properties, Examples
- Amylase Vs Amylose: Definition, Differences, Example