What is Carbohydrates ?
- Carbohydrates are a class of carbonyl compounds (aldehydes or ketones) found in nature that contain numerous hydroxyl groups.
- It could contain derivatives that, when hydrolyzed, produce comparable compounds.
- They’re the typical organic molecules in nature, and they’re also referred to as “saccharides.”
- Sugars are sweet-tasting carbohydrates that dissolve in water.
Structure of Carbohydrates
- Carbohydrate molecules are composed of carbon, hydrogen, and oxygen.
- Carbohydrates have a general empirical structure of (CH2O)n.
- They are chemical compounds with several hydroxyl groups coming off the carbon chain that are structured as aldehydes or ketones.
- Monosaccharides, or simple sugars, are the building blocks of all carbohydrates.
- A polyhydroxy aldehyde (aldose) or a polyhydroxy ketone can be classified as a monosaccharide (ketose).
General Formula of Carbohydrates?
The general formula for carbohydrate is Cx(H2O)y. Although, it must be remembered that this is just a general formula. There are various exceptions to this that we will see. Let us take a look at Acetic Acid which is CH3COOH. Now although this will fit in the general formula of carbohydrate i.e. Cx(H2O)y, we know that acetic acid is not a carbohydrate.
The general formula for carbohydrates is Cx(H2O)y.
Carbohydrates (or sugars) were originally believed to be “hydrates of carbon,” because they have the general formula Cx(H2O)y.
Carbohydrates can be represented structurally in one of three ways:
- Structure with an open chain.
- The structure is hemi-acetal.
- The construction of Haworth.
The lengthy straight-chain type of carbohydrates is known as open chain structure.
In the hemi-acetal structure, the 1st carbon of glucose condenses with the -OH group of the 5th carbon to form a ring structure.
The presence of the pyranose ring structure is known as the Haworth structure.
Properties of Carbohydrates
Carbohydrate Physical Properties
- Stereoisomerism is a term used to describe compounds that have the same structural formula but differ in spatial configuration. In the case of glucose, there are two isomers in terms of the penultimate carbon atom. D-glucose and L-glucose are the two sugars.
- Optical Activity — The rotation of plane-polarized light that produces (+) and (-) glucose.
- Diastereo isomers are configurational variations in glucose involving C2, C3, or C4. Mannose and galactose are two examples.
- Annomerism is the spatial configuration of aldoses in relation to the initial carbon atom.
Chemical Properties of Carbohydrates
- Osazone formation: Sugars react with an excess of phenylhydrazine to generate osazone, which are carbohydrate derivatives. Glucosazone, for example.
- Benedict’s test: Reducing sugars are converted to enediols, which are powerful reducing species, when heated in the presence of an alkali.The colour of Benedict’s reagent solution and reducing sugars changes to orange-red/brick red when heated together.
- Oxidation – If the carbonyl groups in monosaccharides oxidise to become carboxylic acids, they are referred to as reducing sugars. D-glucose is oxidised to D-gluconic acid in Benedict’s test, hence glucose is a reducing sugar.
- Reduction to alcohols:Sodium borohydride, NaBH4, or catalytic hydrogenation (H2, Ni, EtOH/H2O) can convert the C=O groups in open-chain carbohydrates to alcohols. The products are known as “alditols.”
Properties of Monosaccharides
- The majority of monosaccharides have a sweet flavour (fructose is sweetest; 73 percent sweeter than sucrose).
- At room temperature, they are solids.
- They are particularly water soluble: – Despite their high molecular weights, the monosaccharides are substantially more water-soluble than most molecules of similar MW due to the huge amount of OH groups present.
- Glucose can be made into a syrup by dissolving it in a small amount of water (1g/1ml H2O).
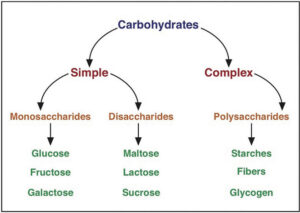
Carbohydrates Classification (Types of Carbohydrates)
Single sugars (monosaccharides) and polymers, oligosaccharides, and polysaccharides are examples of simple carbohydrates.
How to increase Brain Power – Secrets of Brain Unlocked
Monosaccharides
- Since they cannot be further hydrolyzed, they are commonly referred to as simple sugars.
- The material is colourless and crystalline, and it dissolves in water but not in non-polar solvents.
- The term “free aldehyde” or “ketone” refers to compounds that have a free aldehyde or ketone group.
- Cn(H2O)nor CnH2nOn is the general formula.
- They are characterised by the amount of carbon atoms they possess as well as the functional group they include.
- Trioses, tetroses, pentoses, hexoses, heptoses, and other monosaccharides with 3,4,5,6,7… carbons are referred to as aldoses or ketoses, depending on whether they contain an aldehyde or ketone group.
- Glucose, Fructose, Erythrulose, and Ribulose are among examples.
Oligosaccharides
- When oligosaccharides are hydrolyzed, they create 2 to 10 molecules of monosaccharides that are the same or distinct.
- Glycosidic linkage connects the monosaccharide units.
- It is further classified as disaccharide, trisaccharide, tetrasaccharide, and so on, depending on the number of monosaccharide molecules.
- A disaccharide is an oligosaccharide that yields two molecules of monosaccharides when hydrolyzed, while trisaccharides and tetrasaccharides generate three or four monosaccharides, and so on.
- Disaccharides have the general formula Cn(H2O)n-1, while trisaccharides have the formula Cn(H2O)n-2, and so on.
- Examples: Sucrose, lactose, maltose, and other disaccharides are examples. Raffinose and Rabinose are two types of trisaccharides.
Polysaccharides
- They’re also known as “Glycans.”
- Polysaccharides are sugar molecules with more than ten monosaccharide units and can have hundreds of sugar units.
- They produce more than ten monosaccharide molecules when hydrolyzed.
- The identity of recurrent monosaccharide units, chain length, bond linking unit types, and branching degree all distinguish polysaccharides.
- They are primarily concerned with two key tasks: structural functionality and energy storage.
- They’re further divided into groups based on the types of molecules created during hydrolysis.
- They can be homopolysaccharidese (containing monosaccharides of the same type) or heteropolysaccharides (containing monosaccharides of several types).
- Starch, glycogen, cellulose, and pectin are examples of homopolysaccharides.
- Hyaluronic acid and Chondroitin are examples of heteropolysaccharides.
Functions of Carbohydrates
Carbohydrates are a type of chemical found in both plant and animal bodies. Carbohydrates from the skeletal bones of plants and arthropods serve as food reserves in plants and animals. They are a major source of energy for a variety of metabolic functions, and the energy is obtained by oxidation.
Among their main responsibilities are:
- Carbohydrates are used by living organisms to power biological reactions. They are the most abundant dietary source of energy for all living things (4kcal/gram).
- Carbohydrates, in addition to being the primary energy source in many animals, are also immediate energy sources. Glycolysis/cycle Kreb’s breaks down glucose to produce ATP.
- They function as energy reservoirs, fuels, and metabolic intermediaries. It’s stored in animals as glycogen and in plants as starch.
- Instead of proteins, stored carbohydrates provide energy.
- They form structural and defensive components in plant and microbial cell walls. Bacterial cell walls (peptidoglycan or murein), plant cell walls (cellulose), and animal cell walls (chitin).
- Carbohydrates are used as a building block in the production of lipids and proteins.
- Carbohydrates are used as a building block in the production of lipids and proteins.
- Carbohydrates are the brain’s energy source and aid in the regulation of nerve tissue.
- Carbohydrates create surface antigens, receptor molecules, vitamins, and antibiotics when they combine with lipids and proteins.
- The formation of RNA and DNA’s structural framework (ribonucleic acid and deoxyribonucleic acid).
- Many proteins and lipids are connected to them.
- Cell-to-cell communication and interactions between cells and other elements in the cellular environment rely on linked carbohydrates.
- They are a key component of connective tissues in mammals.
- Constipation can be avoided by eating fiber-rich carbohydrates.
- They also aid in the immune system’s regulation.
Click Here for Complete Biology Notes
Carbohydrates Citations
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- Madigan, M. T., Martinko, J. M., Bender, K. S., Buckley, D. H., & Stahl, D. A. (2015). Brock biology of microorganisms (Fourteenth edition.). Boston: Pearson.
- Rodwell, V. W., Botham, K. M., Kennelly, P. J., Weil, P. A., & Bender, D. A. (2015). Harper’s illustrated biochemistry (30th ed.). New York, N.Y.: McGraw-Hill Education LLC.
Related Posts
- Phylum Porifera: Classification, Characteristics, Examples
- Dissecting Microscope (Stereo Microscope) Definition, Principle, Uses, Parts
- Epithelial Tissue Vs Connective Tissue: Definition, 16+ Differences, Examples
- 29+ Differences Between Arteries and Veins
- 31+ Differences Between DNA and RNA (DNA vs RNA)
- Eukaryotic Cells: Definition, Parts, Structure, Examples
- Centrifugal Force: Definition, Principle, Formula, Examples
- Asexual Vs Sexual Reproduction: Overview, 18+ Differences, Examples
- Glandular Epithelium: Location, Structure, Functions, Examples
- 25+ Differences between Invertebrates and Vertebrates
- Lineweaver–Burk Plot
- Cilia and Flagella: Definition, Structure, Functions and Diagram
- P-value: Definition, Formula, Table and Calculation
- Nucleosome Model of Chromosome
- Northern Blot: Overview, Principle, Procedure and Results